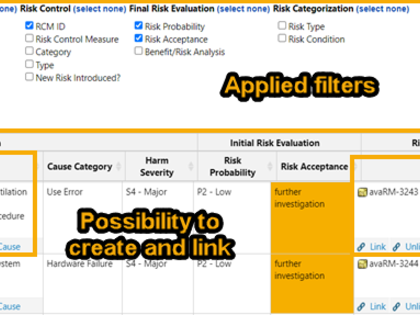
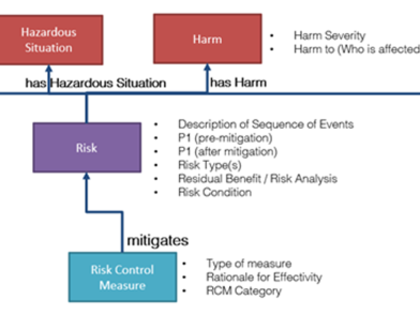
With avaRisk you can easily document your risk analysis in a digital format, link risks directly to the content of interface processes, and visualize dependencies. For instance, defined risk control measures can be linked to design input requirements and a traceability report shows that each measure has been implemented and verified/validated.
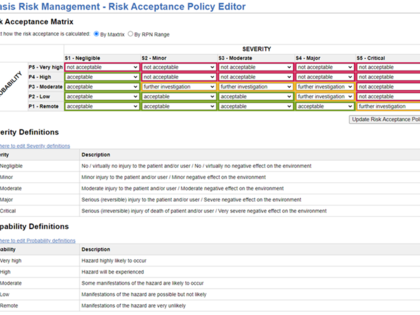
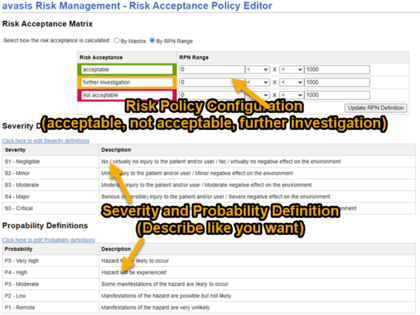
Configurations for different approaches are available for the creation of the risk analysis, e.g. for the identification of risks or the probability of occurrence (P1/P2 and P).
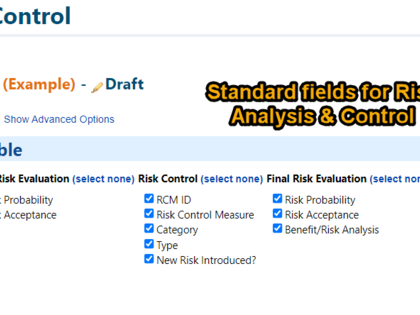
Depending on your individual process, additional functions and attributes can be added, such as thematic grouping of risks (IEC 60601-1, usability, clinical risks), categories of risk control measures, assignment to products or product components.
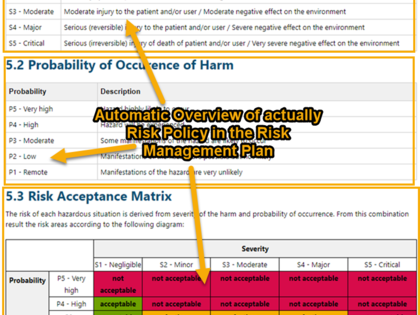
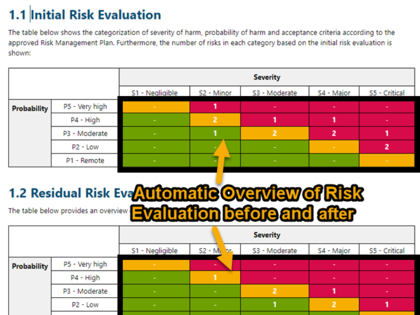
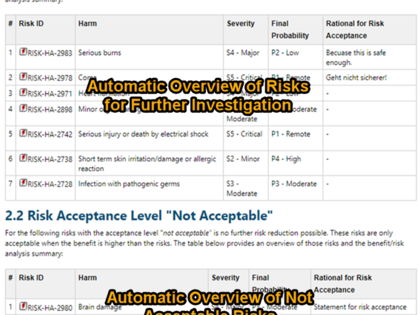
The risk management report includes an automatically created risk summary and various consistency checks to ensure that no inconsistency or incomplete information exists. Information from the benefit/risk assessment of the clinical evaluation can be directly inserted as referenced work items.
avaRisk provides best practice templates based on the requirements of ISO 14971 and can be used immediately. Template content can also be customized by you or can be supplemented with additional features on request.
Overview of the features
- Best Practice Templates according to ISO 14971:2019 for Risk Management Plan, Risk Analysis & Control as well as Risk Management Report
- Easy creation of the risk analysis via guided workflow
- Diverse display and sorting functions of the risk analysis for easy editing
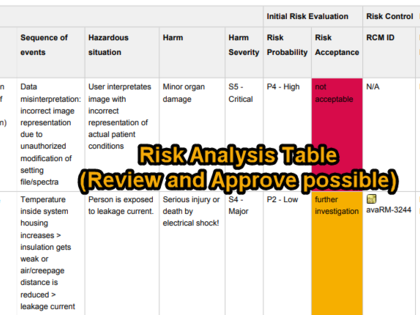
- Methodical variants: Hazard analysis or definition of a combination of Hazard, Hazardous Situation, and Harm
- Variations of the probability of occurrence: P1/P2 or P approach
- Categorization of risks and risk control measures
- Automatic consistency and completeness checks
- Link and traceability reports for interface processes: Development, usability, clinical evaluation, and software tool validation
- Easy reuse of information from documents of interface processes
- Administration of several risk management files Risk files in a Polarion project
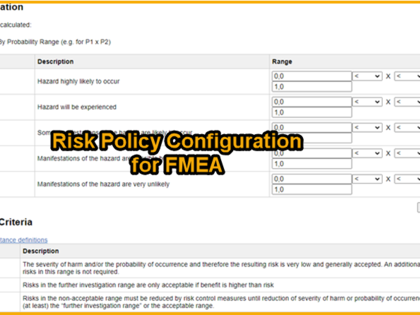
Add-on for the risk analysis according to ISO 14971 through the FMEA method:
avaFMEA is an add-on for avaRisk and offers functionalities for the documentation of an FMEA for e.g. a detailed analysis of the design or production process.
The FMEA is created in the same way as the risk analysis: The user is guided through the dialog to add information.
By linking information, analyses in FMEA tables can be directly linked to corresponding risks in the risk analysis according to ISO 14971.
For more information about avaRisk, please use the following link:
https://www.avasis.biz/en/solutions/avasis-solutions/polarion-medical-device-solutions/avarisk
The avaRisk is one of our best practice configuration packages and is a commercial extension, trial versions can be requested via e-mail, please write to polarion@avasis.biz or use the contact form on our homepage at https://www.avasis.biz/en.