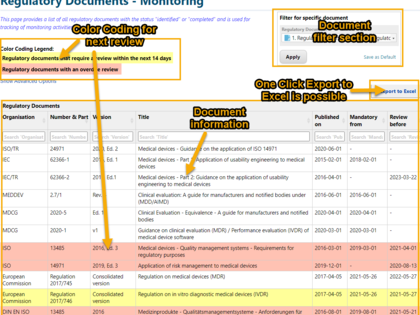
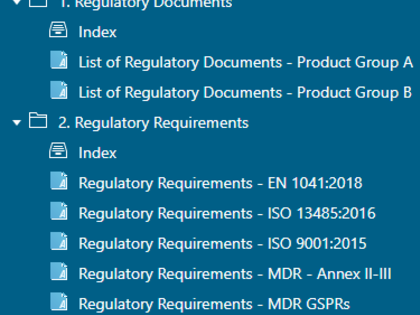
With avaRegulatory you can manage regulatory documents in a central regulatory library, monitor their validity, and provide easy access.
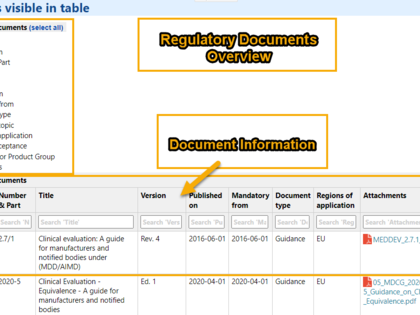
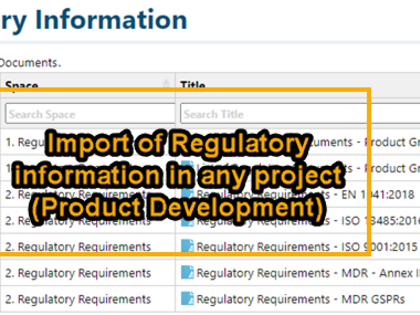
Individual documents can be labeled with additional information and categorized. Filter functions help to find relevant documents quickly, e.g. for further use in product development projects. For this purpose, our solution already offers a number of best practice categories, which can be quickly and easily adapted and extended by yourself. Relevant information for the management review can be compiled as input with one click - manual creation of lists is no longer necessary.
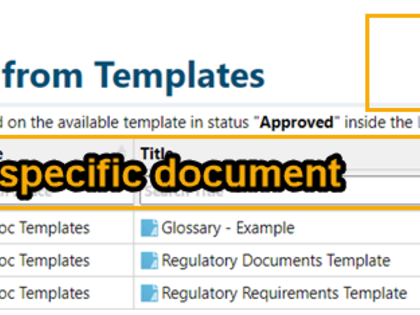
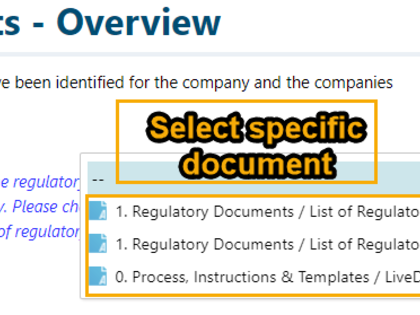
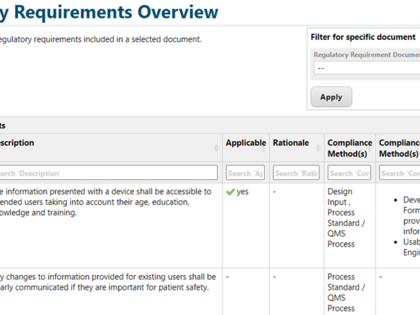
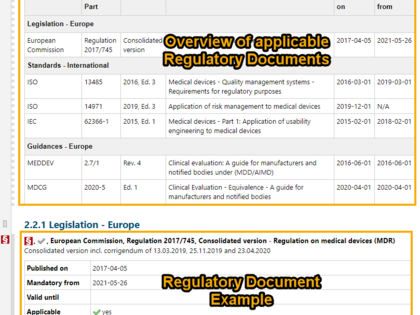
Regulatory documents can be broken down to the individual regulatory requirements and then integrated into product development. For this purpose, avaRegulatory offers special templates and guidance on how you can document and edit content from individual laws, standards, or guidelines in a digital form.
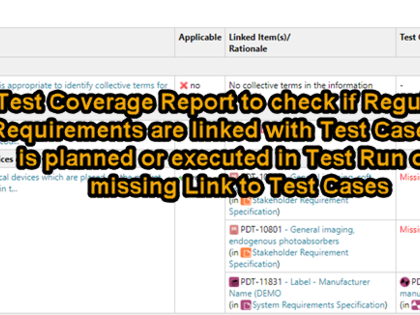
Especially for the medical device and IVD sector, we already offer a template for demonstration of conformity with the General Safety and Performance Requirements ("GSPR") from MDR/IVDR, Annex I. Preconfigured 2- and 3-level traceability reports allow you to demonstrate easily and transparently, how each regulatory requirement has been implemented and what evidence is available to support it.
The standard Polarion functions provide a complete audit trail with which you can document and trace every change.
Overview of the features:
- Central management and monitoring of regulatory documents
- Best practice categories and filter functions enable fast search for further use
- Creation of templates with individual regulatory requirements, e.g. from a specific standard
- Transparent integration of regulatory documents and requirements into product development
- Template for medical device and IVD manufacturers: Digital demonstration of conformity with the Genera Safety and Performance Requirements from MDR/IVDR, Annex I
For more information about avaRegulatory, please use the following link:
https://www.avasis.biz/en/solutions/avasis-solutions/polarion-medical-device-solutions/avaregulatory
The avaRegulatory is a commercial extension, trial versions can be requested via e-mail. Please write to polarion@avasis.biz or use the contact form on our homepage at https://www.avasis.biz/en.