Please do not try to download and/or contact us regarding this extension.
This extension is no longer offered on our portal. This page exists only for historical reasons.
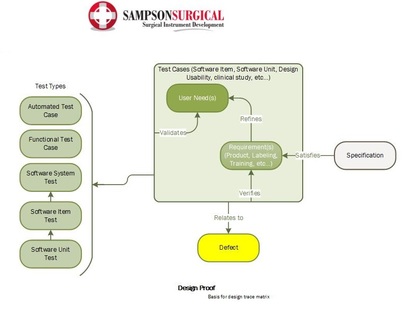
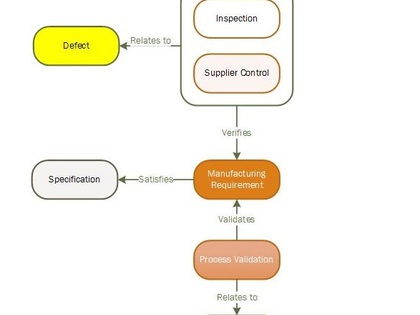
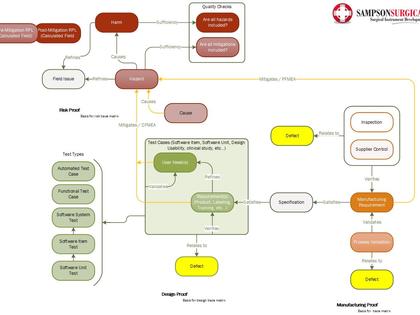
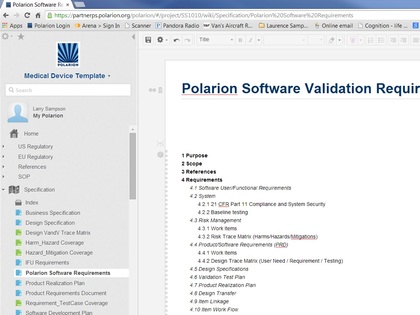
This template was developed for real-life medical device development efforts, and has proven to accelerate workflows, as well as improve the predictability and outcome of the submission process. The template provides the tested framework for an FDA/ISO compliant workflow to help you quickly structure your own custom medical device design controls. Included in the template cost is an hour of consulting time to help you optimize your specific FDA and/or CE submission.
The template is ideal for organizations that are faced with the following questions:
How should we structure our design control for compliance with both CE and FDA requirements?
Polarion is a perfect tool for this task, combined with the template developed by SampsonSurgical, for the express purpose of helping our clients to quickly achieve a cohesive regulatory submission.
What else is needed beside design controls to be compliant with FDA or European medical device regulations?
The FDA requires compliance with a variety of tasks not directly related to design control. Some have to do with registration of the company or compliance with the 21 CFR 820 or MDD quality management systems regulation(s). SampsonSurgical is also familiar with other requirements needed to operate inside the United States, Europe, Austrailia and Canada, and would be happy to assist you with your device regulatory needs.
What are the differences between a CE and a FDA medical device application?
Many. This is a very product specific question. For example, on a class II device the FDA may require only a 510(k) submission process. This type of application is concerned with the “Substantial Equivalence” of a device to a currently marketed predicate. The intent of the design proof is therefore very different than a question of safety or efficacy. SampsonSurgical has a long track record of helping clients to better understand the nuance of this and other submission differences.
How do we best interact with the notified body, or FDA regarding our new device submission?
SampsonSurgical is an expert in the field with a proven track record for a great variety of both FDA and CE submissions. We have good working relationships with many of the FDA review staff, and insight knowledge that has been integrated into the design of this template. We are also available to help you manage your FDA and/or CE regulatory submission process.
Price $495.00
Note: includes 1 hour Consulting